Abstract
Background: The Medicare population, representing older pts with multiple comorbidities, has been successfully treated with CAR T cell therapy. However, prior research suggests that access to this therapy offering possible durable, long-term responses could be limited and subject to health disparities based on socioeconomic status, age, or other pt characteristics (Ahmed et al. Transplant Cell Ther. 2022). Prior research focused on examining determinants of access to CAR T cell therapy relative to a heterogeneous group of pts with respect to treatment history (ie, 0-3 prior lines, etc) or subsequent management (ie, active or maintenance therapy or no additional treatment). The current study examines and contextualizes determinants of receipt of CAR T cell therapy and disparities in access among pts with R/R DLBCL enrolled in a Medicare plan who failed ≥ 2 prior LOTs and were actively treated with ≥ 3 LOTs.
Methods: This retrospective cohort study used the Medicare Limited Data Set (LDS) administrative claims data (from 01/01/2017 to 03/31/2021) containing 100% final action claims submitted for fee-for-service (FFS) Medicare beneficiaries. Claims data were linked to publicly available US census county-level measures of the Area Deprivation Index (ADI) 2019 as well as predisposing, enabling, and need contextual characteristics from the Area Health Resource Files from 2019 to 2020 to measure socioeconomic disadvantage. Pts with newly diagnosed DLBCL (ICD-10-CM: C83.3X) continuously enrolled for ≥ 6 months before diagnosis (baseline period) and who received rituximab (RTX)-based therapy during follow-up were included. DLBCL treatments received by pts were identified using ICD-10 procedure and HCPCS codes; Medicare Part D prescription drug claims were not available. Pts had to have received ≥ 2 prior LOTs after DLBCL diagnosis and initiated a subsequent LOT consistent with NCCN guidelines. Those with prior diagnoses of other non-Hodgkin lymphomas or hematologic cancers were excluded, except for primary mediastinal large B-cell lymphoma, chronic lymphocytic leukemia, and follicular lymphoma. The association between receiving CAR T cell therapy and key patient and county of residence characteristics was assessed using multivariate logistic regression models.
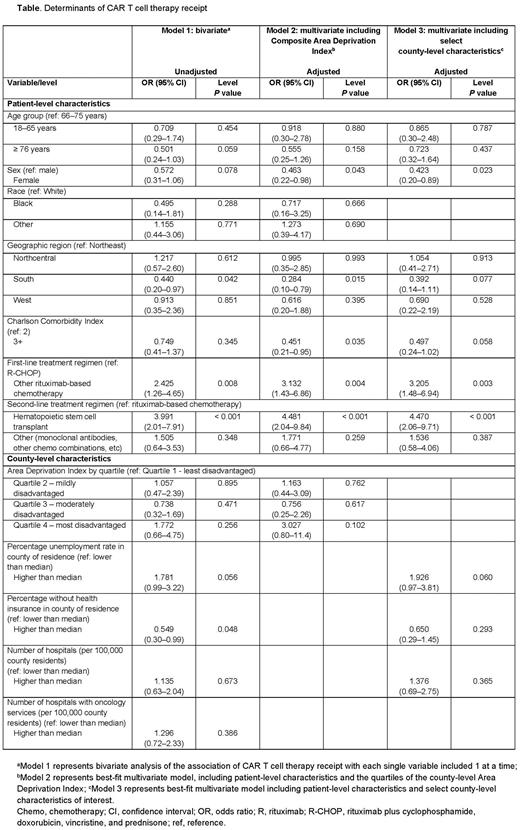
Results: A total of 15,472 Medicare pts with DLBCL received RTX at any point during follow-up. Of those, 266 pts had 2 prior LOTs, RTX in combination with another chemotherapy as first line, and a third or later LOT that was an active comparator of interest (104 pts received CAR T cell therapy vs 162 pts who did not). Among pts who did not receive CAR T cell therapy, the distribution of third LOTs included stem cell transplant (27.8%), polatuzumab-based therapy (26.5%), and various salvage combinations of RTX with chemotherapy (45.7%). More CAR T cell therapy recipients were evaluated for clinical trial participation over the study period versus nonrecipients (37.5% vs 11.1%, respectively). In unadjusted analysis, those ≥ 76 y old were numerically associated with lower odds of receiving CAR T cell therapy vs those 66-75 y, but the difference was not statistically significant (OR = 0.501, P = 0.06). In multivariate adjusted analysis restricted to 209 pts excluding those who were evaluated for clinical trial participation (65 pts received CAR T cell therapy vs 144 pts who did not), CAR T cell therapy recipients were less likely to be female (odds ratio [OR] = 0.463, P = 0.04), have Charlson Comorbidity Index (CCI) score ≥ 3 (OR = 0.451, P = 0.04), or be residents of the South (OR = 0.284, P = 0.02) (Table). There was no association of ADI and receipt of CAR T cell therapy.
Conclusions: Pts with R/R DLBCL on Medicare FFS who received CAR T cell therapy were less fragile, with a CCI < 3, and more likely to experience advanced LOTs. Despite the small sample size of this study, the findings suggest that access to CAR T cell therapy should be particularly improved in female pts and pts living in the South, possibly due to geographical limitations of CAR T centers and a high population of socioeconomically disadvantaged racial minorities. Given the demonstrated durable benefit in pts with R/R DLBCL, increased adoption of CAR T cell therapy can have a significant impact on pts’ lives and improve their health outcomes.
Disclosures
Saunders:BMS: Current Employment, Current equity holder in publicly-traded company. Inguva:BMS: Consultancy. Keating:BMS: Current Employment, Current equity holder in publicly-traded company. Chirikov:BMS: Consultancy, Research Funding; OPEN Health: Current Employment; AbbVie: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal